From NIPT to iNIPT: innovation in prenatal screening
The technology that makes FetalDNA unique in the fetal DNA test landscape is iNIPTTM, developed within the Genetics Laboratory of the Altamedica Healtcare Center in Rome.
It is an integrated platform for the latest generation of genetic sequencing, which involves the synergistic use of technologies of massive DNA sequencing (Next Generation Sequencing) and Digital PCR (Polymerase Chain Reaction).
While the already known method of parallel massive sequencing allows the analysis of the entire genome, Digital PCR represents the possibility of investigating genetic mutations and anomalies with a sensitivity and reliability never achieved before.
In addition, the use of proprietary bioinformatics analysis provides really high reliability in results.
Superiority of iNIPTTM
over traditional NIPT: scientific evidence
It should be noted that, beyond the excessive success rates declared by the traditional NIPT tests on the market for years, their real validity can only be established through Amniocentesis / CVS.
During the period from September 2014 to September 2017, the Altamedica Center in Rome recorded 15,173 invasive prenatal procedures related to Amniocentesis and CVS.
In this large group, we selected 385 pregnancies in which a NIPT test reported a doubt in a chromosomal or subchromosomal abnormality or ultrasound scans revealed the suspicion of a chromosomal or genetic abnormality even if the NIPT test was negative for chromosomal pathology.
This preclinical study showed that:
The first group reported 197 women with a positive NIPT test who underwent an invasive prenatal examination (amniocentesis or CVS) to confirm the diagnosis (TAB 1).
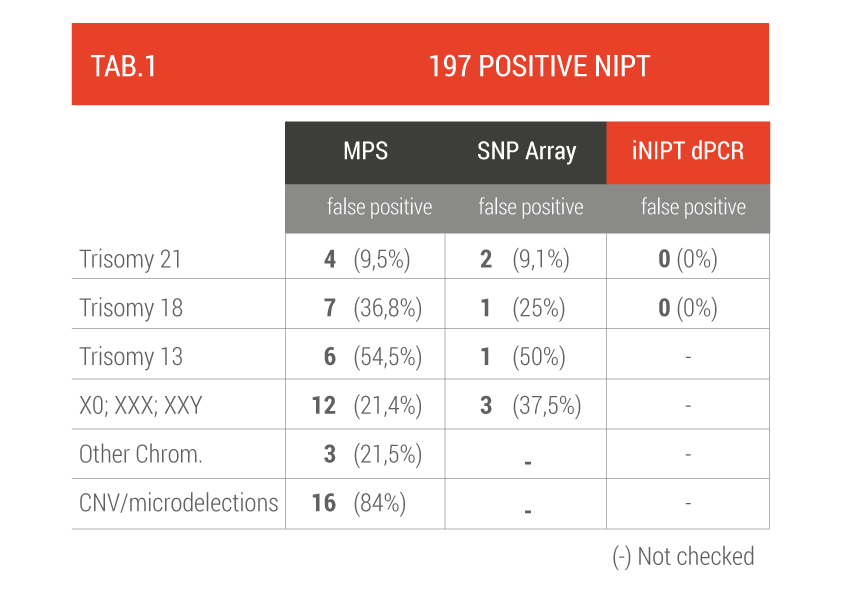
False positives range from 10% to 84% (for microdeletions), 9.5% for T21 and 36.8% for T18, dPCR reduces to zero the false positive rate for fetal abnormalities of chromosome 18 and 21.
Altamedica team performed the FetalDNA test preclinically | iNIPT™ technology to validate its accuracy compared to traditional NIPT.
The second group included 188 women who underwent invasive prenatal diagnosis after a negative NIPT test but there was an ultrasound suspicion of fetal chromosomal pathology (TAB 2).
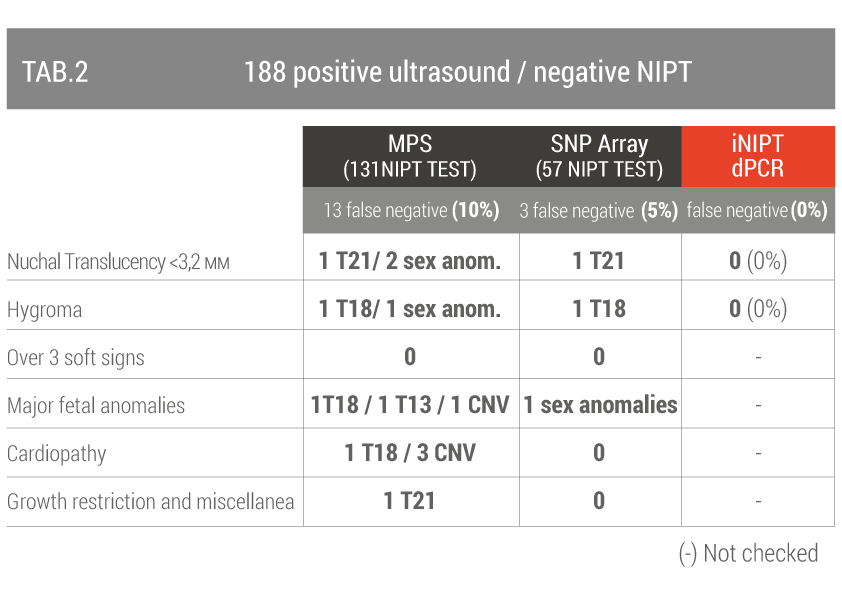
False negatives range from 5% to 10%, dPCR reduces the false negative rate for fetal abnormalities of chromosome 18 and 21 to zero.
The Altamedica team executed the FetalDNA test | iNIPT ™ technology preclinically in order to validate its accuracy compared to traditional NIPT.
* Ultrasound Obstet Gynecol. 2017 Jun; 49(6):815-816. doi: 10.1002/uog.17483. ISUOG updated consensus statement on the impact of cfDNA aneuploidy testing on screening policies and prenatal ultrasound practice. Salomon LJ, Alfirevic Z, Audibert F, Kagan KO, Paladini D, Yeo G, Raine-Fenning N; ISUOG Clinical Standards Committee.